The design of a pharmacy unit within a healthcare facility plays a critical role in ensuring safe, efficient, and compliant medication management.
As a vital component of the clinical infrastructure, the pharmacy is more than just a storage area for medications—it is a highly specialized workspace that directly influences patient outcomes, staff productivity, and operational efficiency.
Core Goals of Effective Pharmacy Design
An optimized pharmacy design is guided by four key objectives:
- Safety: Preventing contamination, medication errors, and staff injuries through controlled environments and ergonomic design.
- Efficiency: Streamlining workflow to minimize wait times, reduce manual tasks, and support seamless coordination with other departments.
- Regulatory Compliance: Meeting stringent requirements from governing bodies such as the USP, FDA, and Joint Commission to ensure medication quality and safety.
- Enhanced Patient Care: Supporting accurate and timely medication delivery, thereby improving the quality of care provided to patients.
If you want to know about the Types of slabs or Permeable concrete or Islamic architecture, please click the link.
1) Introduction
The purpose of the Pharmacy Unit is to provide all inpatient and outpatient pharmacy services including dispensing, preparation of non-sterile and sterile commodities as required, conducting clinical trials as needed, reporting on adverse drug reactions and the provision of drug information and education.
The size and type of service to be provided in the Pharmacy Unit will depend upon the type of drug distribution system used, number of patients to be served, and extent of shared or purchased services. This shall be described in the Operational Policy of the Unit.
Facilities (including satellite, if applicable) and equipment shall be as necessary to accommodate the requirements of the Operational Policy.
If unit dose procedure is used, provide additional space and equipment for supplies, packaging, labelling, and storage, as well as for the carts. Relevant State and Federal statutory requirements are to be complied with.
2) Planning of Pharmacy Unit
i) Operational Models
A Pharmacy may extend its service from a single health care facility to outlying facilities. Specific design requirements for packing, storage and dispatch of goods shall be considered for different operational models.
Unit Dose Systems
The unit dosage system involves packaging of each dose of each medication for patients in a blister pack to provide easy and uniform medication dispensing.
For a unit dosage system, the Pharmacy must include additional space and equipment for supplies, packaging, labelling and storage.
Private Pharmacy
If a private Pharmacy is also to be provided within the hospital’s retail area, the hospital’s operational policy shall determine the type of prescription drugs to be supplied by the private Pharmacy.
It shall also study the impact it has on the main Pharmacy in relation to outpatient dispensing.
ii) Planning Models
Dedicated Outpatient Pharmacy
In facilities where the main Pharmacy cannot be located in a position readily accessible to outpatients areas due to site constraints, then a separate Outpatient Pharmacy may be provided.
This arrangement may result in duplication of services, equipment and support facilities.
Satellite Pharmacy Units
Satellite Pharmacy Units refer to a series of rooms/ suites in a hospital which is remotely positioned from the main Pharmacy and yet managed by the staff of the main Pharmacy.
This may include for example, a dedicated Cytotoxic Unit within a Cancer Day Care Unit or an After-hours Drug store.
Unit / Department – Based Pharmacy Areas
This refers to medication areas located within an Inpatient Unit and may include automated dispensing. Unit based facilities may be located within the Clean Utility or dedicated Medication Rooms in Inpatient Units.
Facilities will include secured drug storage, refrigerated drug storage, space for medication trolleys and computer access for pharmacy personnel.
iii) Functional Areas
The functional areas of a Pharmacy Unit may be sub-divided into two types – “restricted” and “accessible” as follows:
Restricted Areas
- Dispensing Areas which may include separate areas for inpatients and outpatients / ambulatory care patients
- Preparation and manufacturing areas of non-sterile goods
- Active store for imprest stock storage, including assembly and dispatch areas with space allocated for trolley parking
- Bulk stores including unpacking area
- Secured stores for accountable drugs, refrigerated stores and flammable goods storage
- Dispatch area for deliveries to inpatient units
- Drug information areas
Staff areas including Offices, Workstations, Meeting Rooms, Staff Room, Change and Toilets
Accessible Areas
- Reception and Waiting areas for outpatients; it is possible to share waiting areas with an adjoining unit
- Patient counselling and consult areas
- After-hours drug store for access only by authorised personnel and direct entry from outside the main Pharmacy Unit if located within; this room can also be located within a 24-hour zone of the hospital
Optional Areas
Depending on the Role Delineation and Operational Policy, the Pharmacy may also include:
- Sterile Manufacturing, which may include sterile and cytotoxic manufacturing suites, along with support facilities including Anterooms, Change Rooms and Storage
- Facilities for clinical trials, which may include dispensing areas, secured storage and records area and workstations
- Extemporaneous manufacturing area which requires extra space for compounding products
Sterile Preparation Area
Sterile preparation area refers to either Cleanroom facilities housing clean workstations fitted with laminar cabinets or other types of pharmaceutical isolators to meet relevant standard. This includes cytotoxic suites.
Manufacturing Area
The following minimum elements shall be included if manufacturing is performed on-site:
- Bulk compounding area
- Provision of packaging and labelling area
- Quality control area.
Dispensing Stations (Automated)
An automated Dispensing Station may be provided on an Inpatient or Critical Care Unit to dispense prescriptions for patients in that Unit. The Dispensing Station remains under the control of the Pharmacy Unit.
An automated Dispensing Station should be equipped with:
- Automated Dispensing units and refrigerated dispensing units as required; installation according to manufacturer’s specifications
- Shelving for reference texts
- Lighting level adequate for drug preparation areas
- Hand-washing facilities in close proximity
- Bench for drug preparation adjacent to dispensing units
Satellite Pharmacy
A Pharmacy Unit Satellite is a room or unit in a hospital that is located remote from the Pharmacy Unit.
A Satellite Pharmacy requires:
- Bench and sink of stainless steel or other impervious material, supplied with hot and cold water
- Dispensing bench of stainless steel or impervious material; sized according to requirement for dispensing, labelling and packaging
- Computer workstations according to number of Pharmacists in the Satellite unit
- An area for counselling of clients about dispensed or other medicines so that privacy can be assured
- Adequate lighting and ventilation for drug preparation and dispensing
- Air temperature and humidity control suitable to the storage of drugs and medicines
- Handwashing basin and fittings.
The Satellite Pharmacy must be:
- Constructed to prevent unauthorised access by persons other than staff through doors, windows, walls and ceilings
- Fitted with a security intrusion detector alarm that is control room monitored to a central agency on a 24 hour basis.
Storage
The following minimum elements, in the form of cabinets, shelves, and/or separate rooms or closets, shall be included as required:
- Bulk storage
- Active storage
- Refrigerated storage
- Volatile fluids and alcohol storage with construction as required by the relevant regulations for substances involved
- Secure storage for narcotics and controlled drugs
- Storage for general supplies and equipment not in use.
- Storage for prescriptions and any documents required by relevant legislation
iv) Functional Relationships
External
The Pharmacy Unit shall be located for convenient access, staff control, and security. Direct access to loading dock and bulk storage is required if not located within the main Pharmacy Unit.
Internal
Access points provided for the following personnel/ purpose shall be carefully considered:
- Visitors to the Unit
- Pharmacy Staff
- Non-Pharmacy staff to collect prescriptions and medications
- Delivery and prescription collection for outpatients
- Supplies delivery
An interview room for outpatients when provided shall have dual access – separate entries from public area and staff area. Access shall be controlled from inside of the Pharmacy.
Corridors and door openings shall provide sufficient clearance for large items and equipment from bulk stores.
3) Design of Pharmacy Unit
i) General
Design may include provisions for barcode technology for patient prescription identification and tracking as well as electronic prescribing, which will require computer and scanning equipment including additional power and data outlets.
ii) Environmental Considerations
Natural Light
Natural light is highly desirable within the Unit as well as windows permitting outside views. However, such provisions shall not compromise the security of the Unit.
Unauthorised entry and maintaining privacy of the operations of the Unit are the primary concerns in the design of the Unit. Windows shall not permit casual viewing from any adjacent public thoroughfare.
Privacy
Privacy shall be considered in patient consultation areas.
Acoustics
Patient interview and counselling rooms will require acoustic treatment. Please refer to Part C, 9.2 “Acoustic Solutions for Healthcare Facilities”
iii) Space Standards and Components
Ergonomics
Storage systems selected within the Unit shall be accessible to all types of staff. Refer also to Part C of these Guidelines.
iv) Safety and Security
Pharmacy Units and Pharmacies are required to be constructed so as to be as secure as practicable from unauthorised access through doors, windows, walls and ceilings, and to be fitted with a security intrusion detector alarm which is control room monitored to a central agency on a 24 hour basis.
Security measures for consideration will include:
- Electronic door controls
- Movement sensors
- Duress alarms to Dispensing counters
- Security glazing or shutters to Dispensing counters
Accountable Drugs Stores/ Safe shall not be placed in a room positioned on the perimeter of the premises or adjoining a staircase.
v) Finishes
Wall protection shall be installed to prevent damage to walls caused by all types of trolleys. Refer also to Part C of these Guidelines.
vi) Fixtures and Fittings
Refer to Part C of these Guidelines and Standard Components for requirements related to fixtures and fittings.
vii) Building Service Requirements
Refer to Part E of these Guidelines.
HVAC
All drug storage areas shall have temperature and humidity controls; internal room temperature shall be kept below 25oC.
viii) Infection Control
Hand-washing facilities shall be provided within each separate room where open medication is handled. Sterile Suites must have scrub facilities. Refer also to Part D of these Guidelines.
4) Components of the Unit
i) General
The Pharmacy Unit will contain a combination of Standard Components and Non-Standard Components. Provide Standard Components to comply with details in the Standard Components described in these Guidelines. Refer also to Standard Components Room Data Sheets and Room Layout Sheets.
ii) Non Standard Components
Clinical Trials Dispensing
Description and Function – The Clinical Trials Dispensing area will include storage, dispensing, packaging, labelling and records holding for clinical trials drugs. The Clinical Trials facilities will be a separate area within the main Pharmacy.
Location and Relationships – Clinical Trials storage, preparation and dispensing will be located in a separate area within the Pharmacy, and will have ready access to patient Interview and Consultation rooms.
Considerations – Clinical Trials drugs/medications area will require the following considerations:
- Workspace with computer for Pharmacist
- Preparation bench and sink
- Lockable storage for clinical trials drugs, separate from other Pharmacy supplies
- Lockable records storage
- Staff Handwashing basin should be located in close proximity
Aseptic room ( Sterile Manufacturing ) / Cytotoxic room
Description and Function – The Aseptic Room and the Cytotoxic Room are Clean Rooms for manufacturing of medications in a sterile environment. The room will contain laminar flow cabinets and or isolators for sterile manufacturing. The room will be positive pressure and be accessed via an Anteroom. An external outlook is desirable.
Location and Relationships – It shall be located on the perimeter of the facility with an external outlook with access via and Anteroom.
Considerations – The following features shall be considered while designing sterile manufacturing facility:
- Electronic door management system to prevent the opening of both doors in the Anteroom at the same time.
- Handwashing facilities shall be provided immediate outside the Aseptic (Clean) Rooms in adjoining Anteroom; hand basins are not to be located within the Aseptic (Clean) Rooms.
- Provide an intercom system shall be provided between Aseptic (Clean) Rooms and Anteroom
- High-resolution CCTV cameras for remote monitoring
- Comply with room requirements in relevant international Clean Room standards for sterile and cytotoxic manufacturing.
Store – Refrigeration
Description and Function – This can be a room/ bay which consist of multiple refrigerators for storing specific medications. Alternatively, a commercial grade cool room can also be used.
Location and Relationships – This shall be located in proximity to assembly/ preparation area and other storage area within the Unit.
Considerations – Refrigerated storage areas in the Pharmacy will require the following considerations:
- all access doors (either to room or refrigerators) shall be lockable
- temperature monitoring system installed and connect to a centralised alarm/ warning system
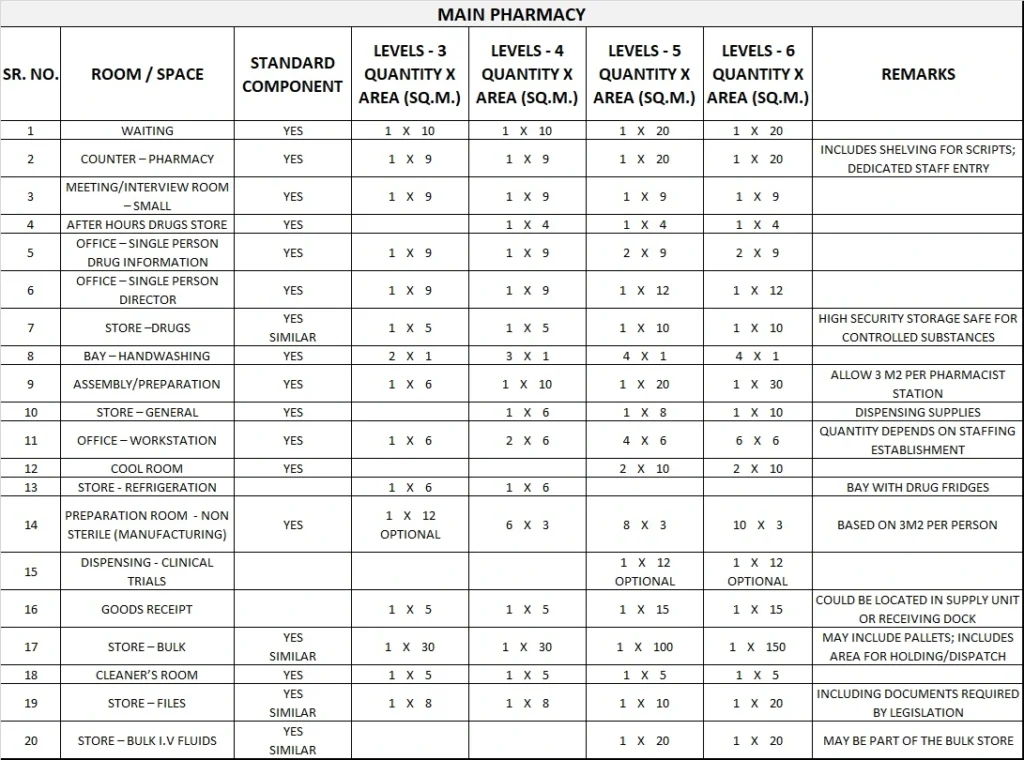
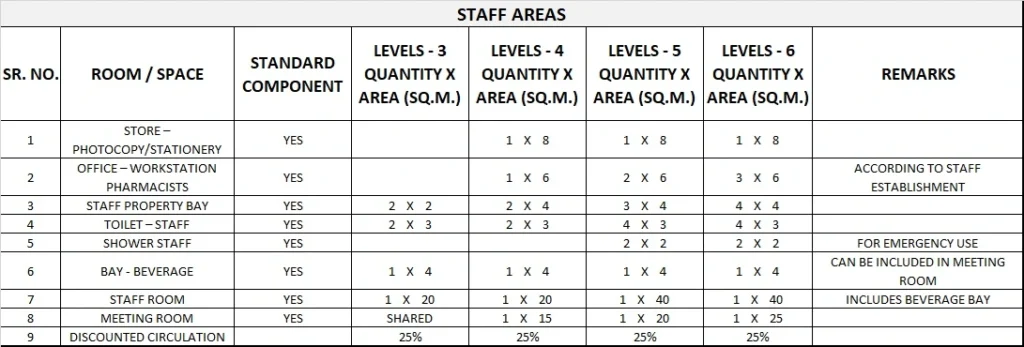
5) Schedule of Accommodation
Pharmacy Unit Generic Schedule of Accommodation
Schedule of Accommodation for a Pharmacy Unit for Levels 3-6
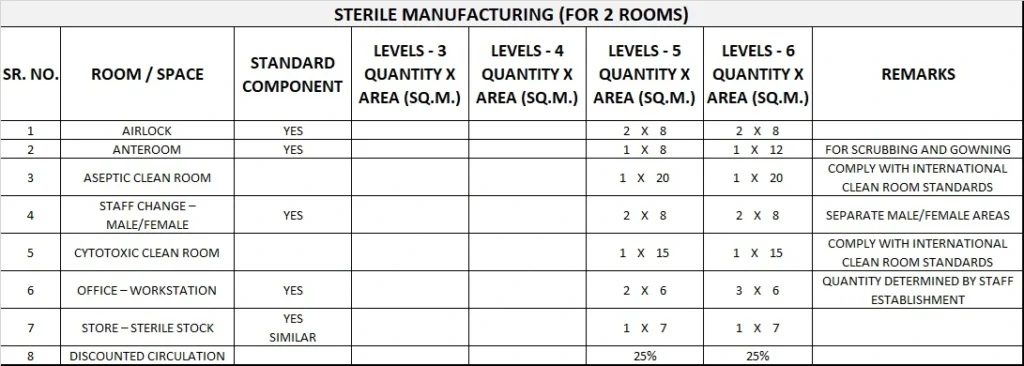
Please note the following:
- Areas noted in Schedules of Accommodation take precedence over all other areas noted in the FPU.
- Rooms indicated in the schedule reflect the typical arrangement according to the Role Delineation.
- Exact requirements for room quantities and sizes will reflect Key Planning Units identified in the service plan and the policies of the Unit.
- Room sizes indicated should be viewed as a minimum requirement; variations are acceptable to reflect the needs of individual Unit.
- Office areas are to be provided according to the Unit role delineation and staffing establishment.
- Staff and support rooms may be shared between Functional Planning Units dependant on location and accessibility to each unit and may provide scope to reduce duplication of facilities.
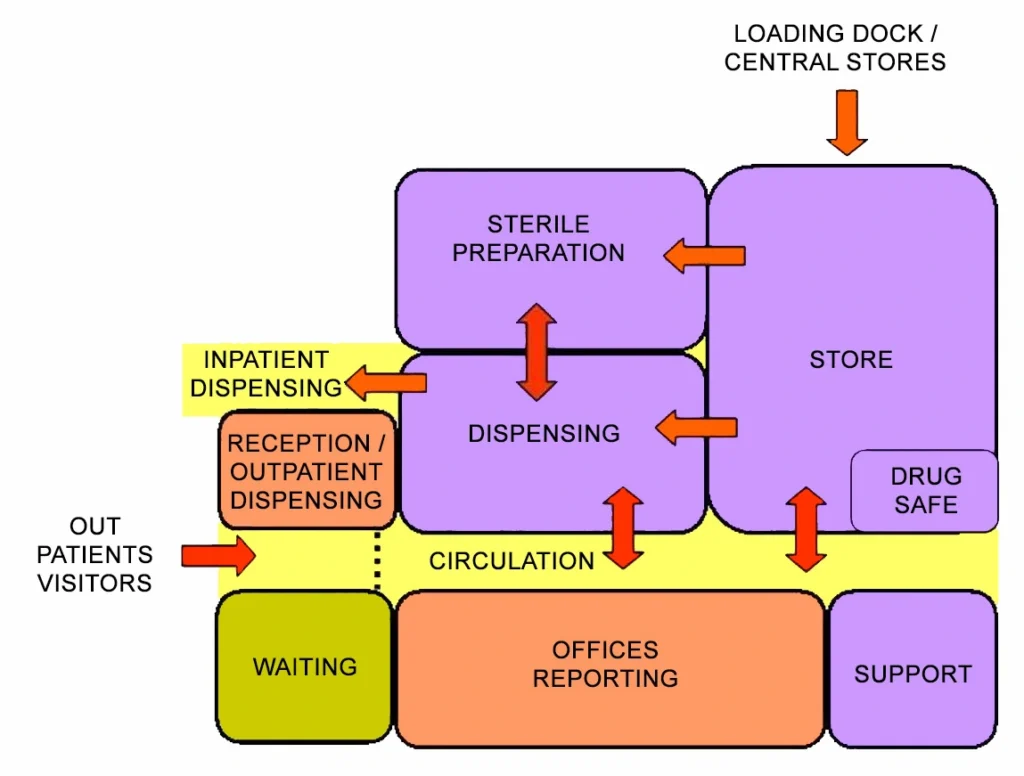
6) Functional Relationship Diagram
Pharmacy Unit Functional Relationship Diagram